DOI: 10.1038/s41598-018-19920-y
- 1. Earth-Life Science Institute, Tokyo Institute of Technology
- 2. Universities Space Research Association,
- 3. NASA Ames Research Center
- 4. Institute for Advanced Biosciences, Keio University
- 5. Spiber Inc.
- 6. Stanford University Department of Bioengineering,
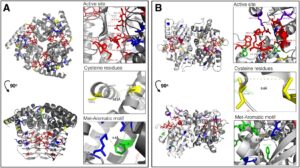
From “Reconstruction of cysteine biosynthesis using engineered cysteine-free enzymes” by Kosuke Fujishima et al.. Licensed undder CC-BY 4.0.
Abstract
Amino acid biosynthesis pathways observed in nature typically require enzymes that are made with the amino acids they produce. For example, Escherichia coli produces cysteine from serine via two enzymes that contain cysteine: serine acetyltransferase (CysE) and O-acetylserine sulfhydrylase (CysK/CysM). To solve this chicken-and-egg problem, we substituted alternate amino acids in CysE, CysK and CysM for cysteine and methionine, which are the only two sulfur-containing proteinogenic amino acids. Using a cysteine-dependent auxotrophic E. coli strain, CysE function was rescued by cysteine-free and methionine-deficient enzymes, and CysM function was rescued by cysteine-free enzymes. CysK function, however, was not rescued in either case. Enzymatic assays showed that the enzymes responsible for rescuing the function in CysE and CysM also retained their activities in vitro. Additionally, substitution of the two highly conserved methionines in CysM decreased but did not eliminate overall activity. Engineering amino acid biosynthetic enzymes to lack the so-produced amino acids can provide insights into, and perhaps eventually fully recapitulate via a synthetic approach, the biogenesis of biotic amino acids.